What Does the H of a Reaction Represent
H H 1 С 1 H 1 0 KOH H - C - OH H Select the correct answer below. Which series of reactions correctly represents chemical reactions related to iron and its compound.

E1 Dehydration Of Alcohols Practice Problems Draw The Mechanism For The Dehydration Chemistry Organic Chemistry Reactions
Tendency toward a former and.

. What does the triangle H of a reaction represent. CHỊ CHOg 250 g 2CO2g 2H2Og Select the correct answer below. The energy required to separate charged particles.
The delta symbol is used to represent change. What does ΔH KJ mean. These reactions are exothermic meaning they give off heat.
Therefore delta H represents the change in enthalpy of a system in a reaction. Resistance or opposition to a force influence or movement especially. What type of reaction does the equation below represent.
Clearly an ATTRACTIVE electrostatic force exists between the charged particles. Moreover you will sometimes see H as well and this symbol on the other hand indicates an unspecified reducing agent. Elimination reaction Substitution reaction.
Well H is the measurement of heat or energy but its a measurement of the transfer of heat or energy. Let us take case by case. A F e C l 2 h e a t F e C l 3 h e a t a i r F e C l 2 Z n F e.
Delta S is entropy. The energy required to separate the charged particles is represented in the equation. What Delta H tells us.
From my previous study I can say O represents an unspecified oxidizing agent like okay you dont need to see what is oxidizing here but rather focus on the products of this oxidation process. O oxidation of aldehydes reduction of aldehydes combustion of aldehyde precipitation FEEDBACK MORE INSTRE content attribution. What does h mean in chemistry.
In chemistry the letter H represents the enthalpy of a system. ΔH for a reaction is equal to the sum of the heats of formation of the product compounds minus the sum of the heats of formation of the reactant compounds. The standard state of water is H 2 O l and not H 2 O g.
H H products - H reactants. H20 - HO H А B Select an answer and submit. N2 3H2 2NH3 a.
H2SO4 H2O HO H A B Multiple Choice O Addition reaction Elimination reaction Substitution reaction. The equation for change in enthalpy is. The Hf for a free element in its state a standard conditions will always equal 0 kJ.
Want this question answered. Enthalpy change Enthalpy change is the name given to the amount of heat evolved or absorbed in a reaction carried out at constant pressure. Assuming a constant pressure a change in enthalpy describes a systems change in.
The units for ΔHº are kiloJoules per mole or kjmol. When delta S is positive and delta H is positive this means that the reaction or physical change is spontaneous at high temperatures. ΔHrxn Σ ΔHf products - Σ ΔHf reactants.
Elimination reaction Substitution reaction. 1pts H2SO4 H20 HO H Addition reaction. 2 What kind of reaction does the conversion of A to B represent.
For keyboard navigation use the updown arrow keys to select an answe a Acid-base reaction b Elimination reaction с Substitution reaction d Addition reaction. Therefore delta H represents the change in enthalpy of a system in a reaction. That means the reaction is.
The oxidation of an aldehyde O the reduction of an aldehyde O a precipitation reaction Previous. Acid Base Salt Water. Its a measurement of randomness or disorder.
In chemistry the letter H represents the enthalpy of a system. Chemistry questions and answers. Hope this helps basically.
The given standard enthalpy of reaction r H indicates the enthalpy change accompanying the reaction when the reactants and products involved are in their standard states. What kind of reaction does the equation below represent. Additionally what is Delta E and Delta H.
The act or process or an instance of reacting. What does Delta S represent. If the energy level of the products is higher than the energy level of the reactants it is an endothermic reaction.
When the product has a greater enthalpy than the reactant then H will be positive. What kind of reaction does the equation shown below represent. An acid and a base react with each other.
A O 2 H 2 O CO 2. We review their content and use your feedback to keep the quality high. Generally the product of this reaction is a salt and water.
Oxygen combines with a compound to form carbon dioxide and water. Be notified when an answer is posted. View the full answer.
What kind of reaction does the conversion of A to B represent. What kind of reaction does the conversion of A to B represent. Enthalpy refers to the sum of the internal energy of a system plus the product of the systems pressure and volume.
It is given the symbol ΔH read as delta H. Xg Delta rarr X e- This reaction represents the enthalpy of ionization of element X.

On The Basis Of The Functional Groups Listed In Chegg Com Functional Group Homework Help Function

How To Quickly Predict The Product Of An Aldol Reaction Organic Chemistry Study Chemistry Organic Chemistry

E1 Reaction Of Cyclohexanes Produces Zaitsev Product Chemistry Chemistry Notes Methyl Group

The Equations Of Physics Represent One Geometrical Process Physics And Mathematics Quantum Mechanics Physics

Ncert Solutions For Class 12 Chemistry Chapter 11 Alcohols Phenols And Ehers Cbse Tuts Alcoho Teaching Chemistry Organic Chemistry Books Chemistry Lessons

Organic Chemistry Educational Infographics Chemistry Reactions Organic Chemistry Introduction To Organic Chemistry Organic Chemistry Reactions

Chemical Formula Easy Science Chemical Formula Matter Science Study Skills

Newman Projection Of Butane Energy Diagram Organic Chemistry Organic Chemistry Study Chemistry Lessons

Pin By M A B Time On Chemistry Ncert H3bo3 Hydrogen Bond Dotted Line
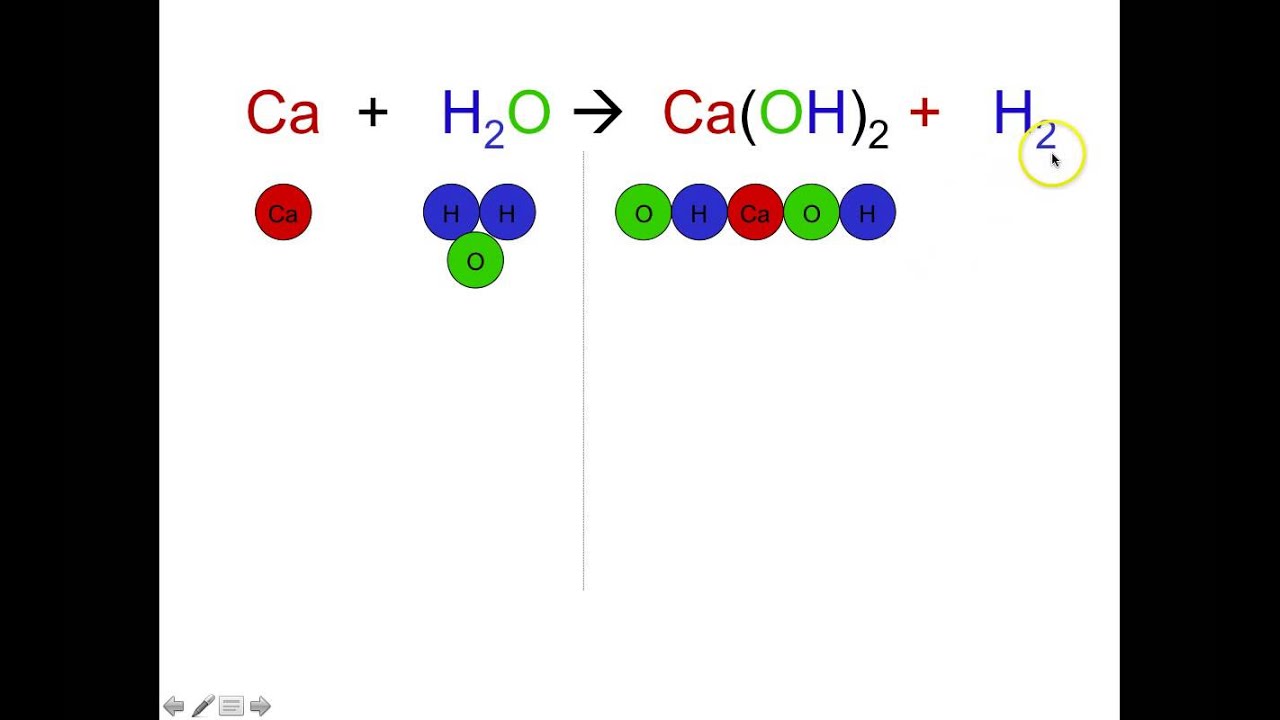
How To Balance Chemical Equations Simple Method For Beginners Chemical Equation Teaching Chemistry Chemistry Classroom

Organic Molecules Playlist On Youtube Organic Molecules Molecules Structural Formula

Sn1 Reaction Mechanism In Nucleophilic Substitutnions Organic Chemistry Chemistry Inductive Effect

Lego Chemical Reactions Lesson Plan Chemistry Lessons Teaching Science Chemistry Lesson Plans

Carbon Protons Neutrons Geometry Arrangment Google Search Ionization Energy Electron Configuration Chemistry

Cytochromes Electron Carriers That Resemble Hemoglobin In The Structure Of Their Active Site The Functional Unit Contains A Central Iron Atom Which Is Capabl

Pin On Sat Chemistry Subject Tests

Pin By Zobaria Kashaf On Chemistry Projects Chemistry Projects Chemical Reactions Chemistry

Meso Compounds Do Not Have Enantiomers Teaching Chemistry Chemistry Lessons Chemistry

Comments
Post a Comment